Volume 3 Issue 1
January 2000
Page
| Discussion of the Artificial Urinary Sphincter (AUS) Dr. Charles Jennings
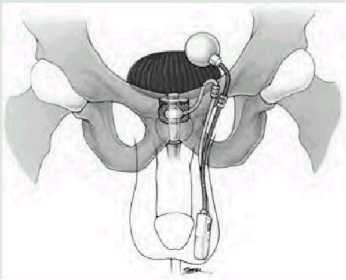
Dr. Charles Jennings is a Diplomat with the American Board of Urology; a Member of the American aUrologic Association; a Fellow of the American College of Surgeons; and a Member of the Society of Urologic Engineering. Dr. Jennings practices in Lima, Ohio. He performed the surgical procedure discussed in this article on Victoria Frohna who, in the following article, describes her experience. The AUS is an implantable prosthetic device that provides an increased pressure resistance to a lower urethral pressure resistance, thus, resulting in urinary continence. The device consists of three connected components (figure). First, there is an inflatable pressure cuff that surrounds the urethra or the bladder neck providing increased resistance against urinary leakage. Second, the cuff is connected to a fluid-filled pressure regulating balloon reservoir that provides the required hydraulic pressure to the cuff. Lastly, a resistor-valve pump is connected to both the reservoir and cuff, and regulates the exchange of fluid between the two depending on whether the cuff is inflated or deflated. The device functions by providing a constant pressure resistance to the bladder outlet from the inflated cuff, thus, providing continence. When this increased resistance is not needed during voiding, fluid is then transferred from the inflated cuff into the reservoir by manipulating the pump, located in the scrotum or labia majora. The cuff remains deflated between two and a half to three minutes, allowing unobstructed voiding. The cuff automatically reinflates through transference of fluid to it from the reservoir via the pump's resistor valve. This process of deflation and reinflation of the cuff is referred to as "cycling."
When bladder pressures attain those greater than urethral resistance pressures, urinary incontinence results. Increased bladder pressures may originate from either its own layered-wall properties or from transmitted abdominal pressures to it due to coughing or straining. Incontinence represents an abnormal relationship existing between the bladder and urethra, either bladder pressure is too high or urethral pressure is too low. As mentioned above, the AUS is generally implanted in patients suffering from urinary incontinence due mainly to this low urethral pressure resistance, provided that they have normal resting bladder pressures. This particular type of incontinence, categorized as Type III, stress variant, may occur after previous surgery involving the bladder neck or urethra resulting in the body's own intrinsic sphincter being incompetent. Another situation where an incompetent intrinsic sphincter may arise is from disorders or injuries of either the spinal cord or its peripheral nerves supplying the bladder neck and urethra. The AUS has its best utility in the setting of an incompetent intrinsic sphincter associated with normal bladder functioning. The candidate for the AUS must be able to completely empty the bladder spontaneously and must not have a resting bladder pressure that is too high. Simple urodynamic testing will identify those patients who either are in urinary retention or carry high resting bladder pressures, both of which threaten the health of the upper urinary tracts and kidneys. Patients who undergo implantation of the AUS for appropriate indications generally fare well as reported in most hospital series. Continence as defined by wearing no protection, such as pads, ranges from 91-92%. Mechanical failure rates for the device in two large series has been reported as 0% and 21%, respectively, with a follow-up range from 2.5-8.9 years. Other less common complications include erosion of the device through the bladder neck/urethra or through the skin, device-related or urinary tract-related infections or, more seriously, upper tract and kidney damage. The implantation of the device, while not technically difficult to perform, requires proper pre-operative preparation including a dexterous, motivated and thoroughly informed patient who has met the appropriate indications for this procedure. The immediate preoperative preparations of the patient include broad-spectrum prophylactic antibiotics along with proper skin and bowel preparations. There should be no deviations from proper surgical technique and the device should be handled very carefully by the OR team. Post-operatively, a foley catheter should be left in overnight. The device should be left in the de-activated mode (cuff deflated) for two to six weeks depending on whether this was a first time insertion or a revision. Revisions usually do not require the longer de-activated time period. All wounds should be kept as clean and dry as possible. Broad-spectrum oral antibiotics should be continued for a few days post-operatively. Anti-spasmodic medications should be provided for bladder spasms that usually occur up to several weeks post-operatively. Light work or recreational activities may be resumed in two to three weeks with return of normal activities, including even intercourse, usually by six to eight weeks. Patients should plan for follow-up visits with their health care providers for as long as they have the device. The visits focus on signs of erosion or malfunctioning of the device, upper urinary tract deterioration, and patient satisfaction. Most insurance carriers will cover the, roughly, $3000 device provided that proper indications for the procedure are documented.
Dr. Charles Jennings
For more information about the AUS prosthetic device:
My Experiences with Transverse Myelitis and Artificial Urinary Sphincter Surgery October of 1997 changed my life dramatically. I had been divorced for a long time, and had raised my two sons alone. Also, I kept very busy with my life as a Licensed Practical Nurse (LPN) at our local community hospital where I had worked 15 years. I recall the last day I had worked, which was October 2, 1997. I also recall the "backache" pain I felt that evening after a 12-hour shift. I felt lucky to have a four-day "off" period, and just relaxed for that period of time anticipating my return to work on October 7, 1997. The morning of October 7, however, it was very clear to me that work was out of the question. I vividly recall awakening at 5:05AM that day, due to the severe pain in my lower back. The pain was very different than previous back pain had been for me. The excruciating pain seemed to start in my low back, but it also literally wrapped around my thighs like a tight band. Due to the difference and severity of that pain, I seriously thought I had ruptured a disc or two in my lumbar spine. That thought was pretty certain in my own mind when I tried to roll over in bed and could hardly move my legs. Somehow, I managed to sit up on the side of the bed and then realized my bladder was very distended or full "up to my earlobes." Holding onto furniture to manage the short trip to the bathroom, I was able to void at the time - for the last time. My attempt to return to bed unsuccessful because of the pain, I screamed for my son who assisted me back to my bedroom just as my legs crumpled beneath me, and I fell to the floor. That morning I did go to work - in an ambulance. My normally low blood pressure was 170/90, due to the pain and also fear regarding my symptoms. I was immediately given Demerol 100 mg, Phenergan 50 mg IM, followed by Valium 10 mg orally. But as I laid on a gurney in ER, even the narcotic medication did little to relieve the pain. My feet and legs were like ice to touch, and the ability to move my feet and ankles was lost. Of course, I was admitted, and a CT scan was done. I received pain meds every four hours, and when I attempted to void again, that was also unsuccessful. At that time I was catheterized. The next day, October 8, 1997, it was decided that I would be transferred to Dayton, Ohio, to be seen by a neurosurgeon there. At a later date, I was told that I was thought to be a "neurosurgical emergency." At the Dayton hospital a MRI was ordered STAT, and done soon after my arrival there. The next morning, another MRI was done, followed by a spinal tap. (I was told that Transverse Myelitis doesn't show up on a CT scan - only on the MRI. Also, my CSF from the spinal tap showed an elevated serum protein - an indication of TM). After the lumbar puncture was completed, my neurologist came to my bedside to tell me the diagnosis: Acute Transverse Myelitis. Never have been so ill in my life. The first week in the acute care facility was difficult for me. After all, I was used to being the "caregiver," and not the patient. The emotional toll felt almost as bad as the physical toll this syndrome had dealt me. From October 8 until October 15, I was given high doses of steroids to help decrease and eliminate the swelling and inflammation around my spinal cord. My urinary catheter was removed and then replaced, after it was determined I had no bladder control. Bowel function was also adversely affected, of course, since my TM injury occurred at the level of L-1. This same week, I did spend some hours sitting up in the hospital room chair - after my caregivers pivoted me into it. At the time, I was unable to bear any weight on my legs. Tearfully, I asked my doctor if "I'll be a paraplegic?" He replied that I would probably be "functional." It was on October 15, 1997 that I was again transferred to a third acute care hospital for "long term rehabilitation." I was told I'd probably be there about six weeks. October 16 was my first full day in rehab. I was actually able to stand between the two parallel bars for the first time. On October 17, I remember my family arriving to visit just after I had walked 36 feet between the parallel bars for the first time! I cried tears of joy, because it was really the first indication that I may actually walk again. Although my right leg was extremely uncontrollable and my left leg was only slightly more controllable, my physical therapists and occupational therapists were also very pleased with my progress. It was necessary to literally tape up my right foot and toes so that I wouldn't trip over my own foot. My right side was more adversely affected because the majority of the swelling was on the right side of my spinal cord - however, the left side was also affected. Initially, I was unable to abduct my right leg at all due to the injury, and standing and keeping my own pelvis beneath me was virtually impossible. By the time I was discharged home on November 7, 1997, (three weeks later), I was elated to be able to stand at a counter for over 16 minutes, and I was walking with the use of a walker a total of 170 feet. My physical therapists asked me if I've always been so stubborn? My answer was, "sure." And I feel that that factor, and especially G-d and the support of family, friends, and co-workers have helped me to cope with this illness so far. It was early in spring of 1998 that I received a call from a nurse/friend of mine who had only recently heard of my illness. My friend told me of a new surgical procedure to eliminate urinary incontinence. The procedure is the implantation of a device known as an "artificial bladder sphincter," and luckily for me there is a local Urologist/Surgeon who is doing the procedure. After examinations, some testing and much discussion, I decided to have the surgical procedure done on April 27, 1998. The artificial sphincter surgery is one of the best things I have done since my onset of TM. The device is relatively simple, with a "balloon" filled with sterile water placed beneath the abdominal muscle near the bladder. The device also has a "cuff" which fits around the bladder neck or urethra, and resembles a very small blood pressure cuff. Then the "pump" of the device is implanted within the labia of a woman or the scrotum of a male. All parts of the mechanism are implanted, and nothing is visible on the body surface, and so only the pump itself can be felt when manipulation is necessary to void. It is very easy and painless to use, and I went literally from 100% urinary incontinence to 100% urinary continence (control) after my surgery, and after healing had occurred. Of course, there is some surgical discomfort after the procedure is done. But the surgical discomfort, at least for me, was minimal as compared to the initial acute pain of myelitis. The sphincter is appropriate for patients like myself, in that I had no bladder control because of the TM. However, I am told that the device is contraindicated in a patient with urinary retention. The best option, of course, is to discuss it with your physician. As I complete this article in early April 1999, my physical therapy is completed. I can walk short distances without my cane (if no obstacles are in my path), but I will need my cane 90% of the time. I also wear an AFO (ankle-foot orthotic/brace) on my right foot and ankle. Balance continues to be a real problem and I fall frequently. Bowel control is also limited as most TM patients are aware, but my bladder is controlled 100% by me. I am hopeful that as time passes, I will regain my balance (and not fall anymore!). I am also hopeful that I can complete my goal of becoming a Registered Nurse - perhaps even working with neurological patients at some later date. Meanwhile, I hope my experience and description of the surgical procedure will help others in similar situations. We only become stronger persons after the adversity we ourselves endure.
Victoria A. Frohna (age 47)
The TMA does not endorse any of the medications, treatments or products reported in this newsletter. This information is intended only to keep you informed. We strongly advise that you check any drugs or treatments mentioned with your physician. |
Go to Next Page
Go to Previous Page
Go to Newsletter Index
Go to Main Page